The life sciences industry has been deprived of digital power for decades. Despite the compelling benefits, the perceived fear of regulations and cost have primarily distanced digital from life sciences firms. Fortunately, this has changed for the better. Regulatory agencies have started advocating digital forms of work as the primary strategy for achieving consistent quality and data integrity. Firms have realized that the return on investment outweighs the cost of ownership. As a result, digital adoption is happening at the speed of light.
Digital maturity in life sciences can be organised in 5 progressive levels:
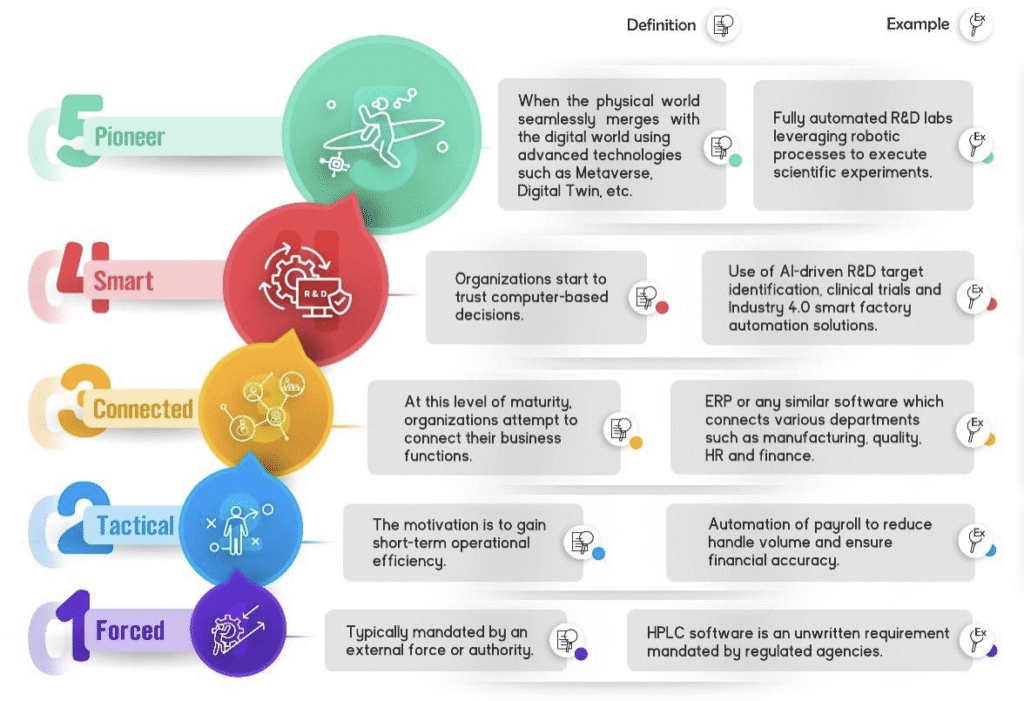
Organizations may be in different level of maturity today. But digitization and automation will soon become a mainstream strategy for achieving agility and consistent quality. For firms overwhelmed about where to start the journey, the following considerations may provide some motivation:
- Re-imagine Quality Systems : A good starting point is to think of how the Quality Management System would change to adopt digital. This will provide the foundation for digital to happen.
- StartSmall:Building confidence early in the digital journey is vital and hence a small success may catalyze and compound into a large-scale digital mission.
- Adopt Open Source : If cost is a constraint, open sources technologies may be considered at the beginning.
The accumulated wealth of digital that life sciences firms were in denial is now available all at once. Among several other benefits, digital breeds peace of mind for businesses. Therefore, it is safe to conclude that there cannot be a more exciting time for life sciences firms to start or advance their journey through the digital maturity continuum.






