Introduction
In pharmaceutical vaccine production, starting, intermediate and end products need to be controlled for the level of antigens. They are tested for the quantity of attenuated or devitalized viruses or bacteria. Since these antigens typically consist of proteins, the analytical quantification of total protein is a method of choice. Pharmacopoeia regulations list a number of relevant methods, among them are several UV/Vis assays (e.g. Lowry, Bradford, BCA assay) and two total nitrogen methods, which is the Kjeldahl and the catalytic combustion method. This application note focuses on the catalytic combustion method which is described in Pharm. Eur. Monograph 2.5.33, Method 7 B: high temperature pyrolysis of the nitrogen compounds in an oxygen atmosphere to nitric oxide (NO) followed by chemiluminescence detection (CLD). This method description almost coincides with the EN 12260, which describes TNb determination in environmental water samples by catalytic high temperature combustion and CLD detection of the formed NO molecules. As this is what multi N/C analyzers are designed for they can be applied for TNb determination in pharmaceutical products as well. The TNb can than be converted into total protein to determine the level of antigens.
Since the nitrogen content of proteins varies, it is widely accepted to convert total nitrogen concentrations into total protein concentrations by multiplying a factor of 6.25 according to the following formula: C [Total Protein] = c [total nitrogen] x 6.25
Materials and Methods
Instrumentation
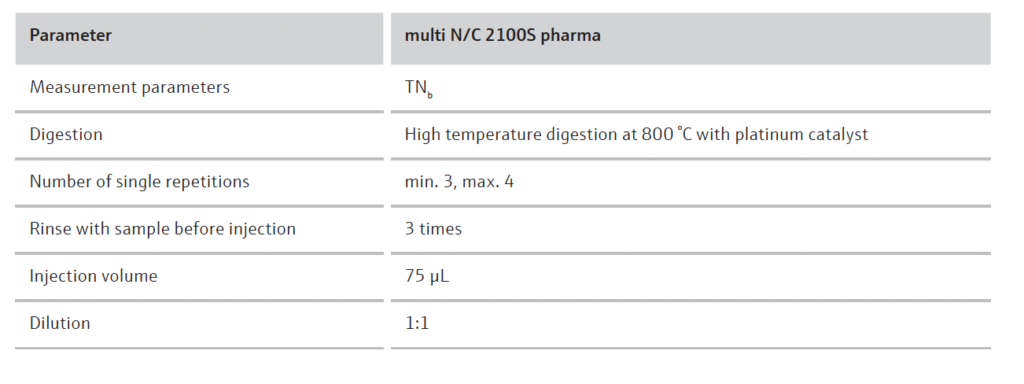
The analysis was performed on the multi N/C 2100S pharma. The method settings shown in Table 1 were used to determine the TOC content.
Five urea TNb calibration standards prepared at customers’ site, one BSA control standard and three unknown customer samples were measured in triplicate determination. The samples were stored at 4 C in the refridgerator until analysis.
After conditioning at room temperature the liquid samples were directly transferred into 2 mL sample vials using a micro pipet and covered with snap-caps. 75 μL of sample aliquots were transferred into the furnace of the analyzer by aid of the micro liter syringe of the autosampler. Supported by platinum catalyst all nitrogen compounds were converted to nitrogen monoxide (NO) in a pure oxygen atmosphere using catalytic high-temperature combustion. The NO was subsequently detected quantitatively by means of a chemiluminescence detector (CLD). The measurement sequence was supported by the auto sampler AS 60 with automatic magnetic stirring of the sampling position and syringe wash station.
Calibration
The multi N/C system was calibrated from 5 to 60 mg/L for total bound nitrogen (TNb) with a multi-point calibration using a BSA protein standard solution. A BSA stock solution of 200 mg TN/L was prepared (Sigma Art.Nr. A-7906, Albumin, Bovine with N-content of 15.60% and purity grade of 98%) weighing 128.2 mg for 100 mL ultra-pure water. The standard conversion factor for such kind of samples (found in literature) for calculating the protein concentration from the measured N concentration of 6.25 was used to calculate the protein concentration of the measured samples.
The calibration curves can be found in Figure 1.

Each sample has been measured at least three times.


All samples were measured with very good repeatability and low RSD’s. The clear advantage of a direct injection system using a micro liter syringe is the short and direct sample transfer from the
vial to the combustion furnace without long tubings and valves. This allows optimized small rinse volumes of max. three times 75 μL. Sample consumption can be kept very low. For five replicates including three rinse cycles and a representative injection volume of 75 μL less than 1.5 mL of sample are required. multi N/C 2100S pharma equipped with a CLD detector for total nitrogen determination is the optimum system to perform total protein analysis for such samples according to the catalytic high temperature digestion method described in Pharm.Eur. 2.5.33 Method 7B.
The recovery of the customer calibration standard solutions and checking standard (BSA), as well as the presented peak shapes of the sample runs, which show no shifts between replicates nor significant peak tailing, proof the performance of the analyzer.






